QMENTA Platform 3.0
Latest release (December 2024)
Version 3.0 includes several major improvements and new functionalities that are critical enablers of success in clinical research and clinical trial execution.
This latest update includes:
Benefits:
- Enable Audit Readiness. Users can easily demonstrate compliance with comprehensive and detailed audit trails capturing every event in data history, including document approvers, status changes, execution of a signature, authentication, and more.
- Improve GCP Quality and Compliance. We improved several features that help assure GCP compliance by the investigator and/or sponsor.
- Improved customer support. The brain is complex enough, you don't need to spend time on IT figuring out how to make things work. We are constantly improving the user experience and how to make the most of our products and services. We have worked on a new knowledge base; included a chat bot to help you quickly find answers; and as always, we are here to help.
Features:
- Custom Electronic Signature and Manifestation. Approve data upload using electronic signatures and manifestations that are compliant with FDA Title 21 CFR Part 11 and Annex 11.
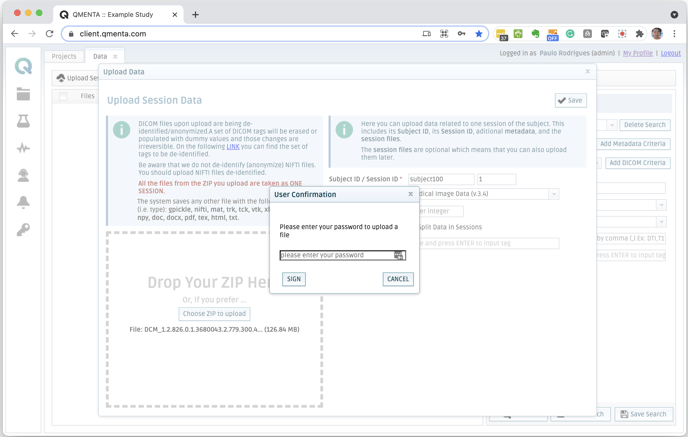
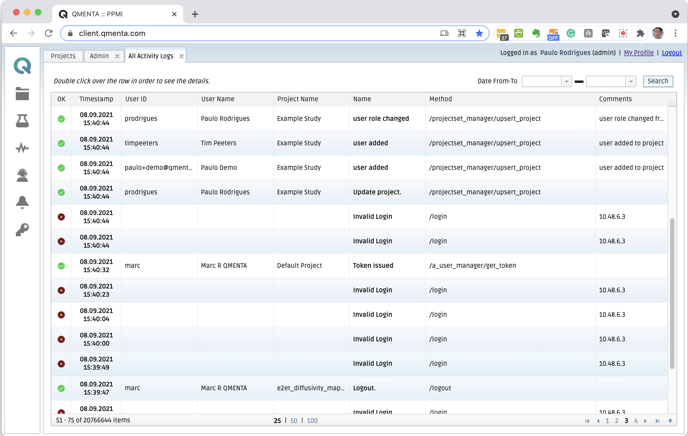
- Comprehensive Audit Trails. Easily demonstrate compliance with detailed audit trails capturing every event in data history, including document approvers and reviewers, status changes, execution of a signature, authentication, and more.
- Two-Factor Authentication (2FA). Besides having Password Security Policies for users to access and provide e-Signatures in QMENTA Platform, 2FA can be enabled requiring users to authenticate via SMS code.
- Brand new unified login/registration experience for all QMENTA solutions.
- General Fixes
Book a demo to know more!
Create free account now!
